Getting behind experimental thermodynamics
Materials research into experimental thermodynamics has been in steady decline for at least 30 years, and is under threat. An international team of researchers assert the field’s importance.

Phase diagrams have been around for more than 200 years. In Alan Prince’s seminal work, Alloy Phase Equilibria, it states that, 'A metallurgist has been defined as someone who thinks in terms of phase diagrams. Whether one accepts this statement or not, it is self-evident that an ability to interpret phase equilibria is essential to the professional metallurgist'.
This statement of course underlines the importance given to this subject back in 1966 when this book was written – the question is whether this statement is applicable today. The science certainly has not changed, what has changed, possibly, is the perception, appetite, or appreciation of the value of the subject.
At its simplest, the physical properties of materials are governed by microstructure, which to a great extent is in turn governed by phase assemblage.
A phase diagram tells the materials scientist what phases are expected to be present under a certain set of conditions of composition, temperature and pressure. Even though, in practice, many materials may be used in a non-equilibrium or metastable (transient) state, the phase diagram provides a crucial foundation and starting point for materials design and process development.
Underpinning the phase diagram are the thermodynamic properties of the materials in question, and once they are known, it is possible to compute the equilibrium or metastable equilibrium states for any set of conditions.
The same thermodynamic properties are routinely used in non-equilibrium situations, such as modelling nucleation and growth processes, the modelling of microstructure, and the simulation of phase transformations. It follows that it is highly desirable to determine these thermodynamic properties.
The field of thermodynamics and phase equilibria is therefore integral to many materials-based industries, through design, processing, process control and, ultimately, recycling. The subject area is growing in stature globally through the advent of relatively new powerful computational techniques (e.g., first principles), often referred to as computational thermodynamics (CT).
Computational solvers/software packages became popular because of their cost effectiveness, by reducing the need for expensive and time-consuming experimental programmes, and the flexibility of use such as working remotely.
Experimental techniques are available, as are ab initio computational techniques, but laboratories that have the experimental capability and technical expertise are scarce – this needs to change. Ab initio computational modelling is not at the stage to easily handle multi-component systems e.g., four-component systems cannot be created with ab initio modelling alone.
Many ternary systems have been studied but to nowhere near the same degree as the binary systems, and many systems are still to be explored. This has resulted in a significant gap in understanding the chemistry of how different elements of the periodic table interact, and thus, has an adverse effect on the materials scientist’s ability to develop new materials or conditions.
This has become particularly important in recent years with developments in open-loop recycling and as materials become more complex with many components. For example, commercial alloys may contain as many as 20 different chemical elements.
It is also very difficult to represent a multi-component diagram in a visual way. But CT allows us to do this, and in principle, there is no limit to the number of components that can be considered. The technique uses critically assessed thermodynamic expressions to calculate the state of chemical equilibrium according to a set of predefined conditions, based on the experimental phase equilibrium and thermodynamic property data for unary, binary, and ternary systems. This enables extrapolation to higher order systems – this is also known as the Calphad technique.
However, software packages must be fuelled by a data format of self-consistent thermodynamic databases that are compiled from fundamental measurements and experimental observation.
Traditionally, the UK has been very strong in this area. UK industry has long recognised the importance of understanding the thermodynamic properties of materials and phase equilibria, and the value of using CT based on them, e.g., steels (British Steel/TATA), nickel-based super alloys (Rolls-Royce), and other materials such as titanium and nuclear materials.
But the importance of the field has not been openly recognised by key institutions such as funding bodies and research councils, partly because the use of thermodynamics has always been associated with highly applied industrial processes, or it was considered to be too fundamental. With the limited recognition and difficulties in securing funding, together with the retirement of experts, there has been a gradual loss of capacity to work in this area.
This will further affect teaching at universities and ultimately leads to a loss of assets/knowledge, which, in turn, affects appreciation of the subject by graduates who then go on to industry with an important and critical gap in their understanding. This consequently will have implications in the long term for UK manufacturing, particularly given the overriding need to rise to the challenges that accompany sustainability.
New materials are being created, but without understanding them fundamentally, it will be difficult to maximise their potential. As we move towards carbon neutrality, having a clear understanding of the thermodynamics associated with any material or process is crucial. Experimental thermodynamics also plays a critical role feeding databases that can enable process digitalisation/autonomy, which is a key UK research and innovation challenge.
Building a thermodynamic database
Generating or compiling critically assessed thermodynamic and phase diagram data for all the potential materials of interest is an enormous task. Even a database for a particular type of material is often well beyond the capability of a single organisation, and so there are numerous examples of collaborative ventures between research organisations spanning many countries to create joint databases.
One such example is thermodynamic databases for lead-free solder alloys, following two European COST Actions that ran from 2002-11. Nineteen countries comprising around 60 research teams were actively involved in the coordinated action studying lead-free and high-temperature lead-free alloys and their interaction with substrate materials.
Studies involved alloy chemistry (phase equilibria and thermodynamic studies, database development, microstructure evolution), physico-chemical studies, and mechanical properties.
Teams from the UK managed to contribute without national funding. Adequate funding for research in this field is crucial if UK institutes are to make a meaningful contribution to global research, especially now that the UK is not part of the EU.
Phase in
It would be easy to present a list of examples that demonstrate how CT can be applied for materials and process development. The casual reader can consult The SGTE Casebook – Thermodynamics at Work edited by Klaus Hack that was published by the Institute in 1996. A few additional examples are shown here for illustrative purposes – the list is not exhaustive and reflects mainly the expertise of the authors.
Cement clinker production
To achieve sustainability in the cement industry, major investments are being made into research to find alternative methods of production. All these methods will greatly benefit from and require an understanding of thermodynamics and phase equilibria in the pyro processing of cement clinker.
Mapping the thermodynamic properties of the mineralogical phases of the clinker as a function of the temperature is essential in understanding clinker formation. This type of modelling requires consistent experimental data. A lack of thermodynamic data is a hurdle to phase diagram model development, hence the need for expertise to build a consistent thermodynamic database focused on cement clinker components.
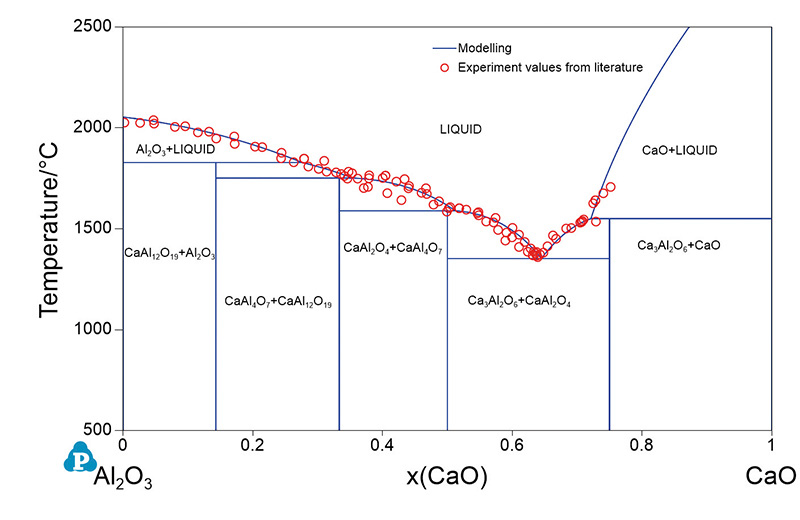
The image below shows the phase diagram of the CaO-Al2O3 system at high temperature computed with Pandat software from CompuTherm LLC. This diagram can tell the manufacturer their target composition and temperature to produce a good quality calcium aluminate cement/clinker. It can also allow the manufacturer to troubleshoot any change in quality/composition and reveal what changes can be made to move away from the undesired processing window to the appropriate process ‘goldilocks zone’ – which in this case will be close to the melting point in the CaAl2O4+CaAl4O7 region.
Metallurgy
Phase diagrams have long been used in metallurgy to predict the effect of processing and to tailor the properties of final products. New approaches to alloy design involve using calculated data from thermodynamic databases as input data for machine learning and integrated-computational materials engineering, making quality thermodynamic data critical for rapid alloy development.
The use of phase diagrams and prediction of materials behaviour is also vital for alloy development for high-temperature applications, such as turbine engines, nuclear fission, or fusion reactors – allowing the industries to avoid difficult or costly experimental works.
The importance of quality thermodynamic data has also been highlighted with the rapidly expanding area of high entropy alloys (HEAs). HEAs typically have five-plus components in near equal amounts. Due to their composition, they are stable within areas of a phase diagram that are often not well studied, which significantly delays their development. Reliable phase diagrams for high-order systems are highly desirable, and CT would make a great contribution to this, together with necessary additional experimental data to accelerate the HEA design.
For example, refractory niobium-silicide-based (Nb-Si) alloys are proposed as next-generation materials for turbine engines as blades or disks. These alloys have high melting points and excellent high-temperature strength, and therefore could increase the efficiency of turbine engines, reducing emissions by allowing the engines to run hotter. These alloys however do suffer from oxidation.
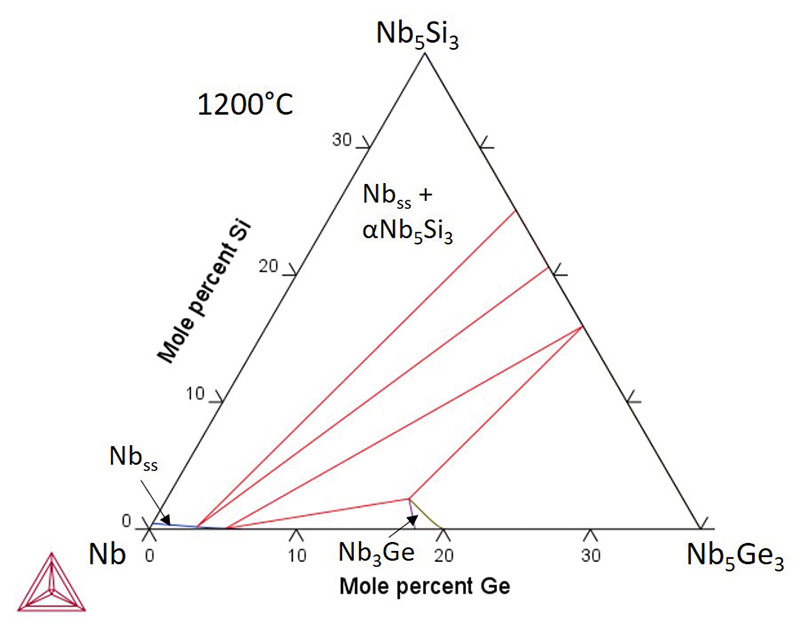
Alloying additions can improve properties, and in this case, adding germanium to an Nb-Si alloy is of interest to increase the oxidation resistance. To model the impact of adding germanium, a thermodynamic phase diagram description has been generated using the Calphad method (see image right).
An isothermal section at 1,200°C shows the calculated region with desirable phases (Nbss and ɑNb5Si3). This database was used with other thermodynamic descriptions to calculate the effect of germanium on phases in complex alloys and, when combined with an oxide database, to predict the oxide phases formed at high temperatures – allowing the alloy composition to be optimised to improve oxidation performance.
Lighting
The development and manufacture of commercially successful light sources has always relied upon understanding the physical, chemical and materials interactions within the devices and processes. This has become ever more important in the era of light emitting diodes and organic light emitting diodes. For example, an understanding of the phase diagram and equilibrium thermodynamics of a multi-component system has been essential in Ceravision Limited, UK, developing a manufacturing process for an electrodeless metal halide discharge lamp.
It is necessary to introduce milligramme quantities of an amalgam into the arctubes. Use of a gallium-indium-mercury (Ga-In-Hg) liquid amalgam, plus liquid Hg and a solid mercury (II) halide was the preferred combination for this application.
However, the Ga-Hg system is known to have a miscibility gap which is expected to extend into the Ga-In-Hg system. This has been confirmed by experiments, most notably Gubbels paper on Phase equilibria in the Ga-Hg-In system. To ensure the variations in lamp performance are within the required tolerances, it is essential that the liquid amalgam is homogeneous. If not, non-reproducible results are assured. Hence it is important to understand the range of compositions and temperatures where the miscibility gap in the Ga-In-Hg system is avoided and only one liquid phase is present.
A Calphad modelling of the ternary Ga-In-Hg system has been undertaken using accepted thermodynamic descriptions of the associated binary systems along with the experimental information on the ternary available in the scientific literature. By selecting compositions based on the model of the Ga-In-Hg system, a reliable and reproducible manufacturing process was established. The liquids are introduced into the arctube via precisely controlled microlitre-syringes.
Establishing the UK’s position
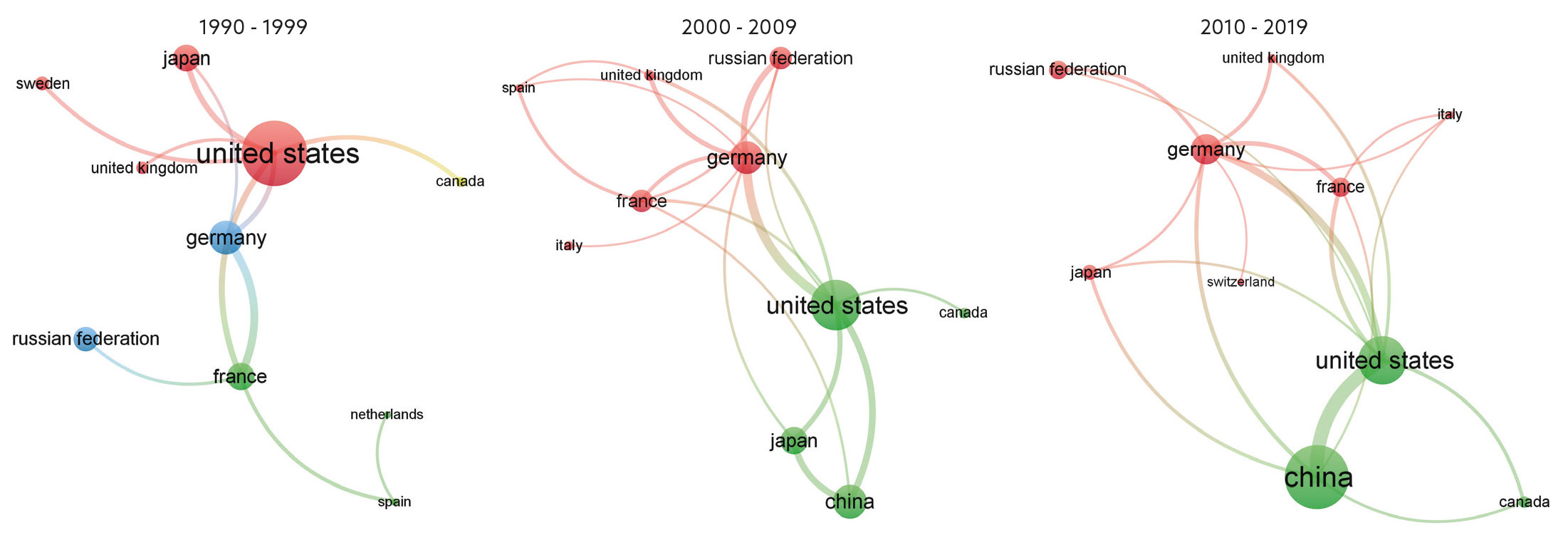
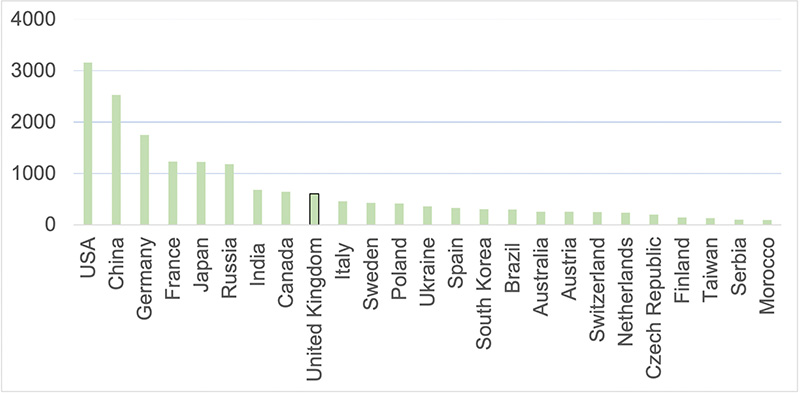
To understand the UK’s current position, publication metrics data from Scopus are assessed. The image below shows that the UK is currently ninth in output in the field. In the other image above, the size of dot shows the decrease in UK expertise – the UK used to have strong links with the US in this area, but main partners in the field are now the major EU countries and Russia. With current political turmoil, including Brexit and the Russo-Ukrainian war, the UK needs to establish a critical mass in this area of expertise or could fall further behind economically.
If no action is taken to restore expertise in the area, the effect on UK industry could be immense. An overreliance on ‘blackbox’ commercial databases will occur, further reducing understanding of thermodynamics. Lack of teaching and leaders in this area will lead to a lack of ‘next generation’ talent, and UK graduates will be less able to compete in global markets.
Several consequences are envisaged including:
- Irreplaceable loss of expertise
- Organisations seeking advice from outside the UK
- Decline in the UK manufacturing industry
- Decrease in resource efficiency through wasteful (material and energy) processes
- Knock-on damage to the environment
Turning the tide
So how can the UK restore its position as a major contributor in this field? The answer may be to increase facilities and expertise within the UK. There has been a general decline in ability to conduct basic measurements and very little Calphad modelling undertaken within the UK. But this can be only part of the solution, the problems go much deeper than that.
There is a lack of appreciation throughout the UK materials community of what the basic understanding of phase equilibria, through modern modelling techniques, can achieve. To a great extent, this seems to be left to the commercial software providers who can give blackbox solutions to a myriad of problems.
However, someone must provide the basic information to the black box. Reliance on this black-box approach without understanding the data behind it can cause severe loss and damage.
But there is a glimmer of hope. The Department of Materials Science and Engineering at the University of Sheffield is establishing a Centre for Experimental Thermodynamics. It currently houses a high-temperature (up to 1,600°C) Calvet calorimeter that can perform high-temperature (up to 1,500°C) measurements by isothermal drop calorimetry for accurate heat capacity, as well as heat content and high-temperature (up to 1,600°C) measurements by heat flux differential scanning calorimetry (temperature scanning conditions).
These are the types of experiments needed to generate thermodynamic data, creating new databases and extending existing ones. It should be pointed out that this equipment is unique in the UK. It is ironic that, in the 1990s, two similar calorimeters were housed in Sheffield – sadly, both long since consigned to the ‘scrap-heap’ with declined interests in the field.
So how do we further improve the situation? This must start in the universities and backed by the institutions such as IOM3. The teaching of phase diagrams and thermodynamics needs to be strengthened, and Calphad modelling should be an integral part of that, in lectures, practical classes and projects. Furthermore, we call on funding bodies to acknowledge that expertise within the UK of thermodynamics and phase equilibria is in the national interest and we seek research grants that allow this expertise to be nurtured and the development of necessary fundamental work.
If interested in the topic, you can join the emergent IOM3 Materials Chemistry Subgroup under the Materials Characterisation & Properties Group. For more information, contact [email protected]







