Enhancing water-repellent properties
Researchers in Finland claim to have created a surface that demonstrates exceptional water-repellent qualities by depositing a liquid-like coating on a nanostructured silicon substrate.
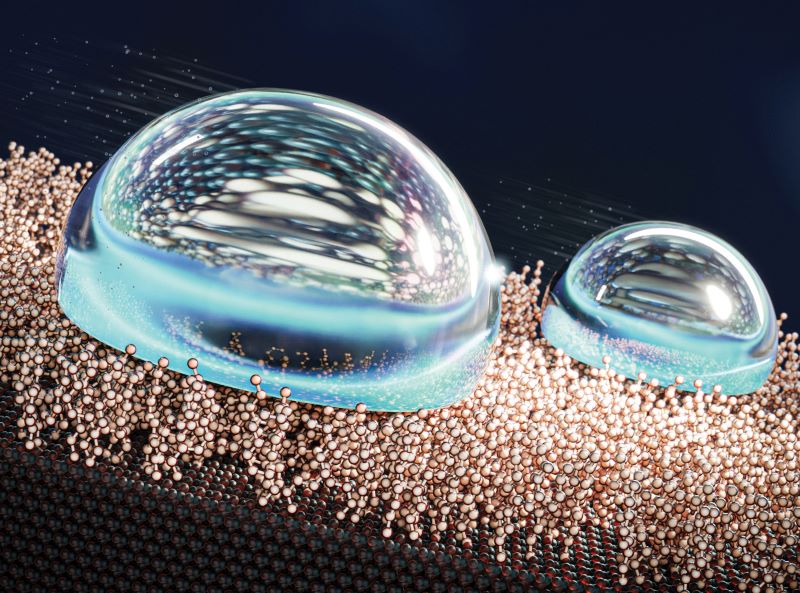
The 1nm-thin, liquid-like layer of non-fluorinated molecules acts as a lubricant and tests show water droplets simply slide off the surface.
The researchers from Aalto University in Helsinki believe their discovery challenges existing ideas about friction between solid surfaces and water, and also opens up new possibilities in the study of droplet ‘slipperiness’ at the molecular level.
'It was presumed that heterogeneous surfaces lead to friction of droplets,' says Professor Robin Ras, who spearheaded the research. 'That has been demonstrated for rough surfaces, or where you have topographical heterogeneities. It has also been demonstrated with chemical heterogeneities at the micrometre scale, but it has never been demonstrated for chemical heterogeneities at the molecular scale. Here, we have a method to really control molecules at a single layer down to molecular scale.'
Ras says the chemical coating they have created on the nanostructured surface offers a wide range of potential industrial applications. He adds the team is currently making demo samples for companies.
'Because these coatings are so thin, they are also transparent to light, and many applications such as camera lenses will require optical transparency.
'These coatings could potentially help keep surfaces clean and functional and, if they are kept clean, they would also need less maintenance.'
The liquid-like layer of molecules, known as self-assembled monolayers (SAMs), are created in a specially designed atomic layer deposition reactor that also uses a light source and a detector.
'We just take one molecule and deposit it on the surface and the chemistry makes sure that it forms no more than a monolayer,' Ras explains.
'Just by increasing the time that we allow this molecule to react with the surface, we increase the density of these molecules, so more and more are deposited, and they get closer to each other, covering a larger part of the substrate.'
By making careful adjustments to the reactor’s internal temperature and its water content, they can gradually change the water contact angle from around 10° to 110°, thereby fine-tuning how much of the silicon surface is covered by SAMs.
An operando spectroscopic ellipsometer is also used so the scientists can study the SAMs’ growth in detail, adds Ras.
Tests show that when there is very high deposition of hydrophobic SAMs, the water droplets display a spherical shape that is similar to Teflon and they slip off the surface easily.
The team did not expect to see a similar level of slipperiness for the low-coverage hydrophilic SAMs. In this case, the water droplet makes large contact with the silicon surface, but also exhibits exceptional slipperiness.
'When we tilt the surface a few degrees, the droplets just glide off. That was very peculiar and we had not expected this,' says Ras.
According to the team, water only sticks to the silicon surface in between the low and high SAM deposits.
Sakari Lepikko, the study’s lead author, notes that because the SAM is nanometre thin and disperses easily after physical contact, the next research step is to study how its durability can be improved.
Lepikko says, 'Things like heat transfer in pipes, de-icing and anti-fogging are potential uses. It will also help with microfluidics…Our counterintuitive mechanism is a new way to increase droplet mobility anywhere it’s needed.'







