Element focus CARBON.pdf
The Basics
-
Carbon has atomic number 6 and atomic mass 12.0107. It sits at the head of group 14 between boron and nitrogen and with silicon beneath.
-
Carbon and has four electrons in its outer shell, leading to carbon’s ability to form more compounds than any other element – more than 10 million!
-
Although carbon is only the 15th most common element in the Earth’s crust, after hydrogen, helium, and oxygen it is the 4th most abundant element in the observable universe.
-
It could be argued that we have been living in the carbon age since the dawn of time. Carbon is fundamental to our very being and its use, in its many forms, has underpinned advances in our technology for millennia.
-
From its first uses as a fuel in the form of wood, to the many and varied uses today, carbon impacts everything we do.
-
Carbon has five naturally occurring isotopes of which C12 and C13 are stable and C14 has a half-life of 5730 years.
-
Carbon-14 is produced when cosmic ray neutrons interact with nitrogen atoms and these radioactive carbon atoms are found consistently at a concentration of 0.0000000001%. The decay of C14 is used to date ancient materials and Carbon Dating has been described as one of the most significant discoveries of the 20th Century.


Structure & Properties
-
Carbon has 3 naturally occurring allotropes, amorphous, graphite and diamond. In the early 1980’s a fourth form, Buckminster fullerene, was discovered and in 2010 Andre Geim and Konstantin Novoselov from the University of Manchester won the Nobel Prize for Physics for their work isolating and characterising a fifth form, graphene.
-
Amorphous carbon includes soot and coal which have little or no crystal structure.
-
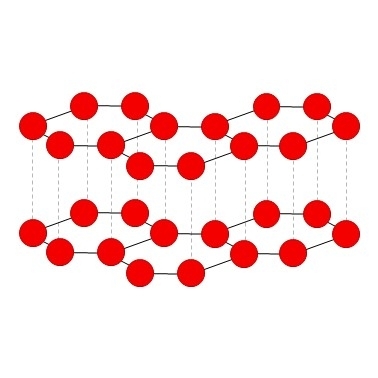
Graphite is the most stable allotrope of carbon has a hexagonal layer structure and is very soft, as the bonds (Van der Waals forces) between the layers are very weak.
-
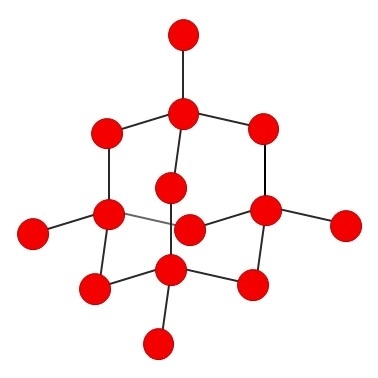
Diamond has a tetrahedral structure and is produced naturally when carbon deposits in the Earth’s crust are compressed under enormous pressures at high temperatures in a process that takes thousands of years.
-
Fullerenes, spherical structures, usually containing 60 carbon atoms arranged in hexagons and pentagons like a football, are hollow molecules can be used for holding atoms.
-
Graphene is a sheet of carbon only one atom thick. These sheets can be manipulated to create structures with interesting chemical, physical and mechanical properties, that it is hoped will eventually find many uses.
Materials & Applications
-
Carbon is crucial in the manufacture or iron and steel. In the form of coke (processed from coal), it is the reducing agent in the blast furnace and varying quantities of residual carbon give steels their fascinating microstructures and useful properties.
-
Graphite is used in pencils and as a lubricant. It also conducts electricity, so can be used as electrodes in industrial electrolysis processes.
-
Diamond is the hardest substance known and as such it is used for polishing and machining other materials. Its chemical inertness and wear resistance allow it to be used in jewellery. Man-made diamonds can be made by replicating this process and these are mainly used in abrasives and cutting equipment.
-
Carbon forms many thousands of organic compounds. These include gases such as methane, butane and propane which are used for cooking, fuels such as petrol, diesel and the myriad of thermoplastics that have found applications in everything from food packaging to prosthetic limbs.
-
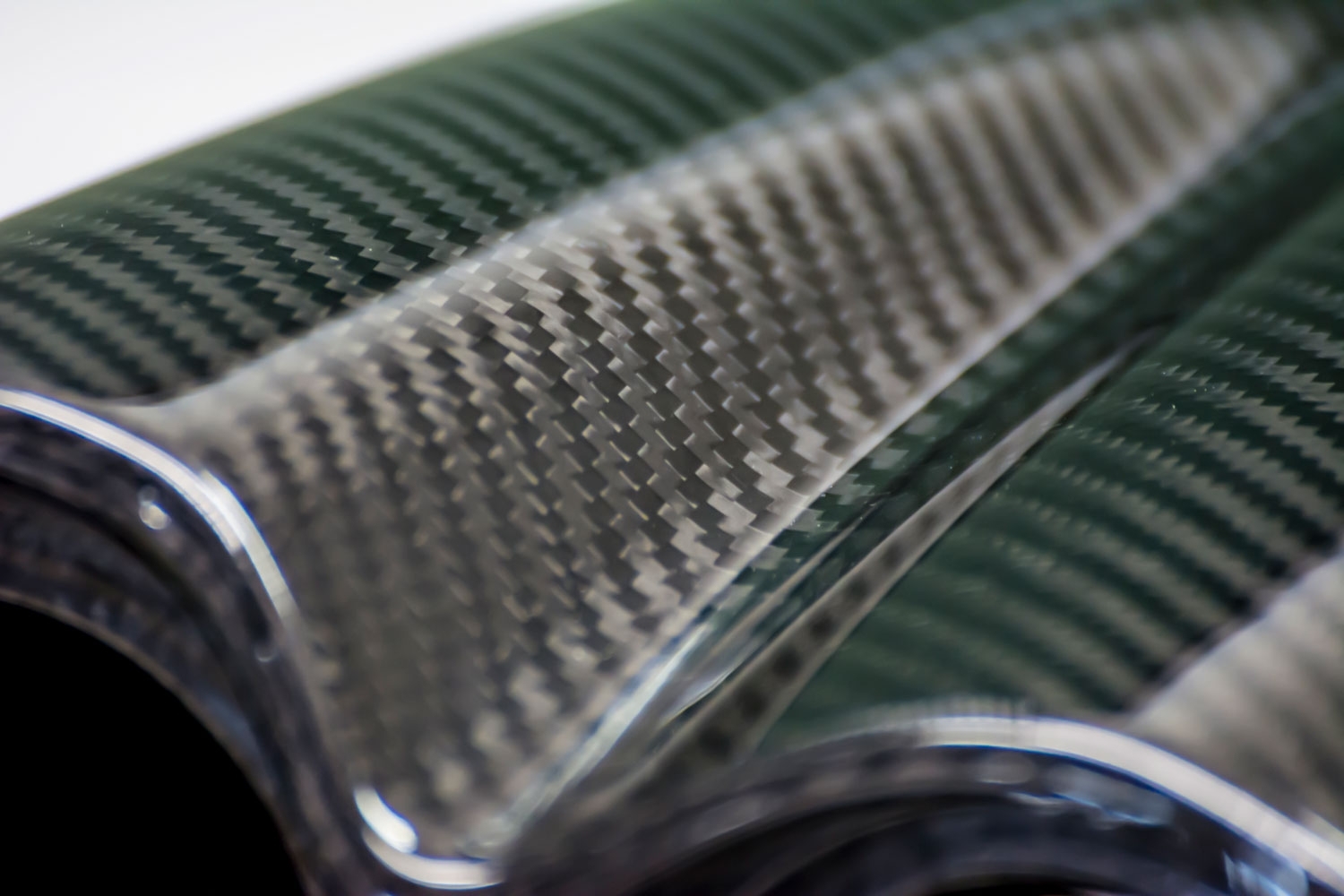
Carbon fibre composites (CFC) have become very popular in recent years as they have a high strength to weight ratio. The fibres can be oriented to give maximum strength exactly where it is required. They have been used in aircraft to reduce weight and improve fuel efficiency. The vertical tail and fixed training edges of the Airbus A380 wings are made from CFC, as is the whole fuselage of the Boeing 777. Composites are also used extensively in sport (fishing rods, bicycles, Formula 1 cars, squash and tennis racquets etc.), medical and automotive applications.
-
A carbon-carbon composite is used as a heat shield in reusable space craft and in braking systems in high performance cars due to its high heat capacity, low coefficient of thermal expansion and its ability to retain its mechanical properties up to a temperature of 2000°C. It is comprised of carbon fibres embedded in a matrix of graphite.