Atomically thin boron for practical use
New material borophane is stable outside a vacuum, opening possibilities for real-world applications.
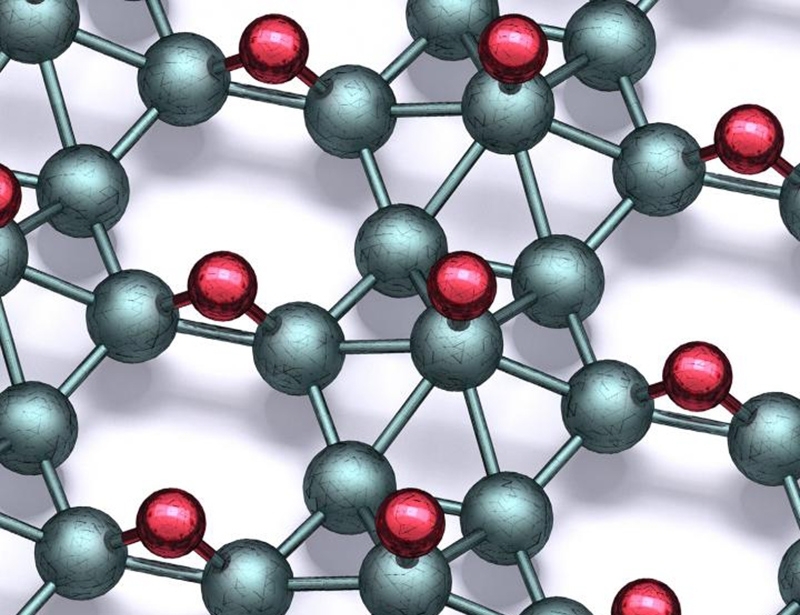
Northwestern University researchers, USA, have, for the first time, created borophane -- atomically thin boron that is stable at standard temperatures and air pressures.
Researchers have long been excited by the promise of borophene -- a single-atom-thick sheet of boron -- because of its strength, flexibility and electronics properties. Stronger, lighter and more flexible than graphene, borophene may change batteries, electronics, sensors, photovoltaics and quantum computing.
However, borophene immediately oxidises in air, losing its conductive properties, so cannot exist outside of an ultrahigh vacuum chamber. This has severely hampered the exploration of borophene and its properties.
By bonding borophene with atomic hydrogen, the Northwestern team created borophane, which has the same exciting properties as borophene and is stable outside of a vacuum.
The research has been published in Science and marks the first time scientists report the synthesis of borophane.