A tough cell? Safeguarding hydrogen vehicles
Katharine Coggeshall, Science Writer at Los Alamos National Laboratory, USA, reports on a contamination detector that can safeguard hydrogen fuel cells.

Hydrogen fuel cells offer the promise of clean and sustainable energy for transportation to emergency back-up power. The only emission when converting high-purity hydrogen fuel to energy is water vapour.
However, adoption of this clean-energy technology is not as widespread as it ought to be. Advances to reduce technology costs and to safeguard fuel quality are needed.
Contaminants such as carbon monoxide, sulphides and ammonia (among others) can infiltrate hydrogen fuel through various avenues – through the process of creating the hydrogen fuel, or while the hydrogen sits in storage. Regardless, once there, the contaminants follow the fuel to fuelling stations, where consumers refill their hydrogen-powered vehicles. That fuel heads to the vehicle’s fuel cell to be converted to energy. The conversion process requires a platinum-based catalyst, and it is here that contaminants do their harm.
The contaminants can chemically interact with and bond to the metal catalyst, rendering it inactive. Without the catalyst, there is no conversion and the vehicle stops. Of course, the level of contamination matters, and low levels may not lead to an inoperable vehicle, rather it leads to a non-optimal one, given that constant exposure to low levels can potentially shorten the life of the fuel cell. In the end, the fuel cell may need to be replaced.
Consumer confidence
For widespread adoption of hydrogen-powered vehicles, consumers need to have confidence that fuel quality will comply with standards, including those designated by the Society of Automobile Engineers (SAE) in the USA.
There is an obvious financial burden that accompanies contaminated hydrogen fuel. Companies might want warehouses without toxic exhaust harming enclosed workers and clean-energy trucks that don’t require the downtime of charging (like electric options), but they also want peace of mind when investing in hydrogen-powered vehicles. They do not want to risk the ill effects that come from low-purity hydrogen fuel.
Up until now, hydrogen-fuelling stations have relied on expensive analytical instruments that require constant maintenance or deliver a delayed response if gas samples are sent off-site for testing – a burdensome process and one that is not particularly incentivising financially for station managers or indeed those customers footing the bill.
Canary in a coal mine
An electrochemical hydrogen contamination detector (HCD) has been built at Los Alamos National Laboratory (LANL), from the same technology employed in hydrogen fuel cells and stacks – multiple cells. Both technologies use a proton-conducting polymer electrolyte membrane (PEM) material, which completes the electrochemical circuit between the anode and the cathode. PEM material is already low cost and manufactured on a large scale. The HCD specifically uses a Nafion electrolyte membrane and electrodes made from platinum (Pt) or Pt-ruthenium (Ru) catalyst particles on porous gas diffusion layers. Platinum is expensive, so, for HCD, the Pt is sputtered in small quantities onto the diffusion layers, as shown by the schematic below.
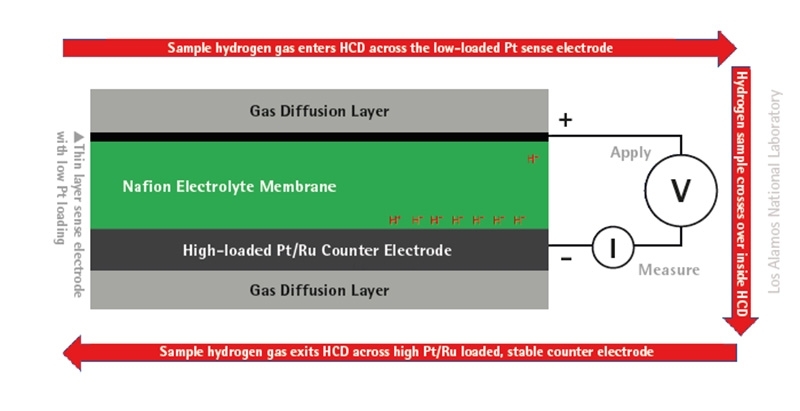
The key here is synonymous poisoning – if a hydrogen fuel cell is damaged by a given contaminant, so would the HCD. In this way, the HCD is like a canary in a coal mine, alerting people to something toxic before severe damage can be done – detecting contaminants in hydrogen fuel before it can damage a vehicle’s fuel cell using basic electrochemical techniques.
The device can specify which contaminant is the issue and at what concentration. This is particularly important as the threshold for true contamination will vary depending on contaminant species. The platinum catalyst is hypersensitive to impurities in the hydrogen gas stream. Therefore, the hydrogen fuel sample is pumped through the device via the electrochemical pump and directed across the Pt electrode and then past a Pt-Ru counter electrode. Since this electrode is similar to the one used in a fuel cell, albeit hypersensitised, any contaminant that impacts the performance of a fuel cell electrode will cause a dramatic decrease in hydrogen pumping current measured by the HCD.
LANL has partnered with Skyre Inc., in Connecticut, USA – a firm that specialises in hydrogen and carbon dioxide conversion – to develop a HCD model that can accurately measure contamination.
The partners entered a Technology Commercialisation Fund, supported by the Office of Technology Transfer and the U.S Department of Energy’s Hydrogen and Fuel Cell Technologies Office, part of the Office of Energy Efficiency and Renewable Energy. In 2020, they released a pre-commercial model of HCD, which was tested in a hydrogen fuelling station at H2 Frontier, a consultancy that serves hydrogen project applications in California, USA.
In trials, contamination has been detected down to the parts-per-billion (ppb) level. HCD has easily detected carbon monoxide at 200ppb, the SAE maximum permissible standard level for this impurity in the field – with laboratory testing demonstrating detection as low as 10ppb. Detecting carbon monoxide at this level of sensitivity allows the user to monitor the situation in near-real time, before truly contaminating levels are reached in the fuel. The testing data has also been verified by the National Renewable Energy Laboratory (NREL) in the USA.
Sulphides have also been identified in laboratory testing, with similar results anticipated for ammonia. However, carbon monoxide and hydrogen sulphide are the two most critical contaminants to protect against in real-world use.
Forget about it
The ‘set and forget’ aspect of HCD is likely the most appealing benefit. The only maintenance required is intermittent water re-supply for the patented internal gas sample humidification system, which permits sampling of dry hydrogen gas at the station directly without the need for any pre-treatment. This re-supply is simple and rapid, however, another version that does not require re-supply is already being investigated.
The new HCD model will use a proton-conducting membrane that does not require internal humidification to efficiently operate in dry hydrogen streams. This will remove the need for water re-supply and allow the system to operate at the 700-bar refuelling pressures typically used by most filling stations without a step-down in pressure.
Beyond sustainability
The cost of the current pre-commercial HCD is $3,000 per unit and is anticipated to fall below this cost in production. It is hoped scale-up can be achieved with Skyre Inc., which it has also patented with, and H2 Frontier Inc. its field testing partner. It is realistic that every hydrogen fueling station could afford the HCD device, and multiple units could be installed at a given station.
It is powered by a computer USB port and runs continuously, so hydrogen purity status can be accessed online. If a contaminant threshold is crossed, the station manager will be notified almost immediately. As an additional precaution, the alarm can be tied directly to a station dispenser cut-off for around-the-clock protection.
Consumers will not adopt hydrogen fuel simply because it’s better for the environment, it has to be financially feasible as well. Replacing a fuel cell stack every time low-purity fuel makes its way into the vehicle is not sustainable, so removing the risk is critical.







