Systematisation of substances and materials 2.0
Why might systematisation of materials be useful above current groupings?

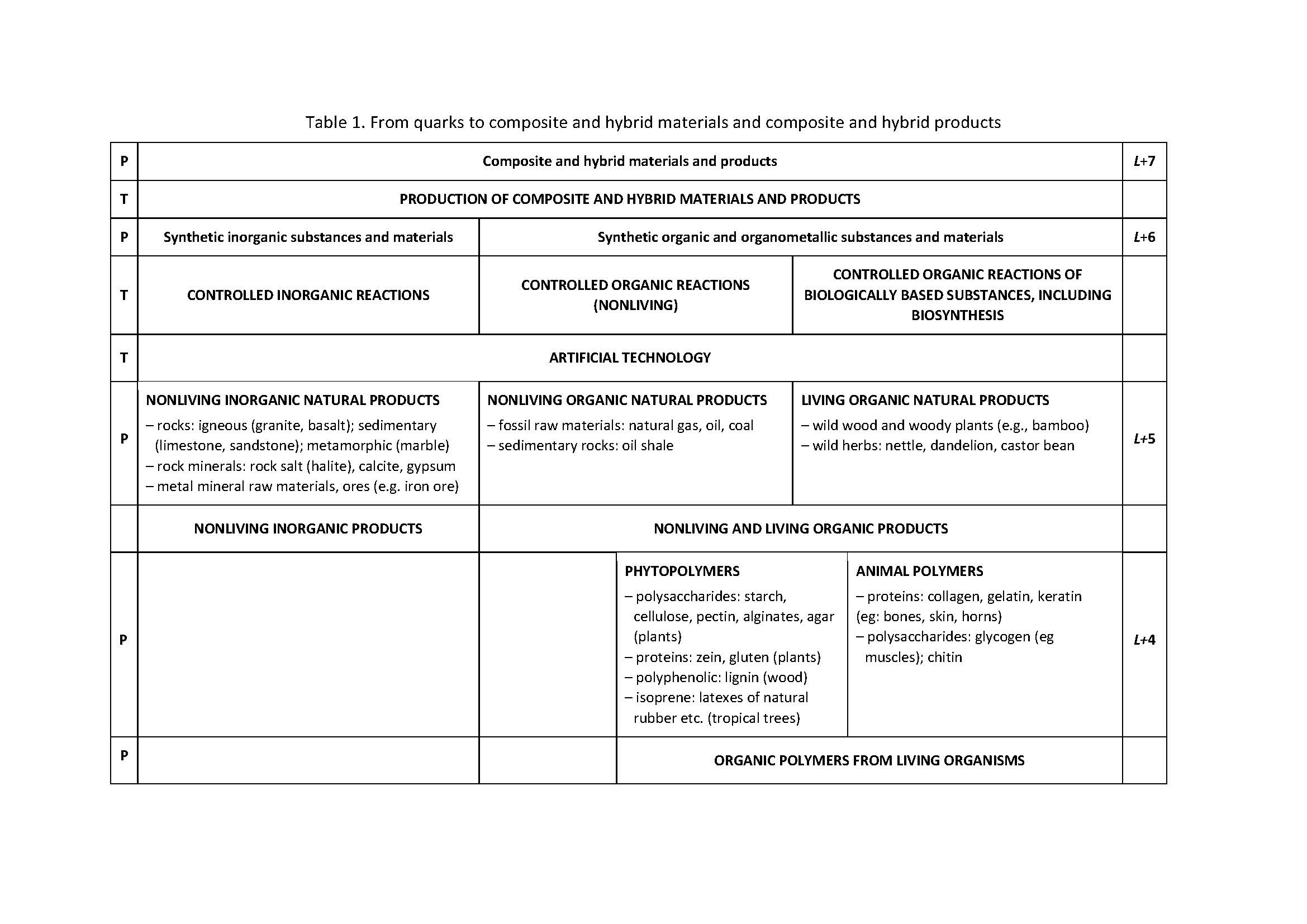
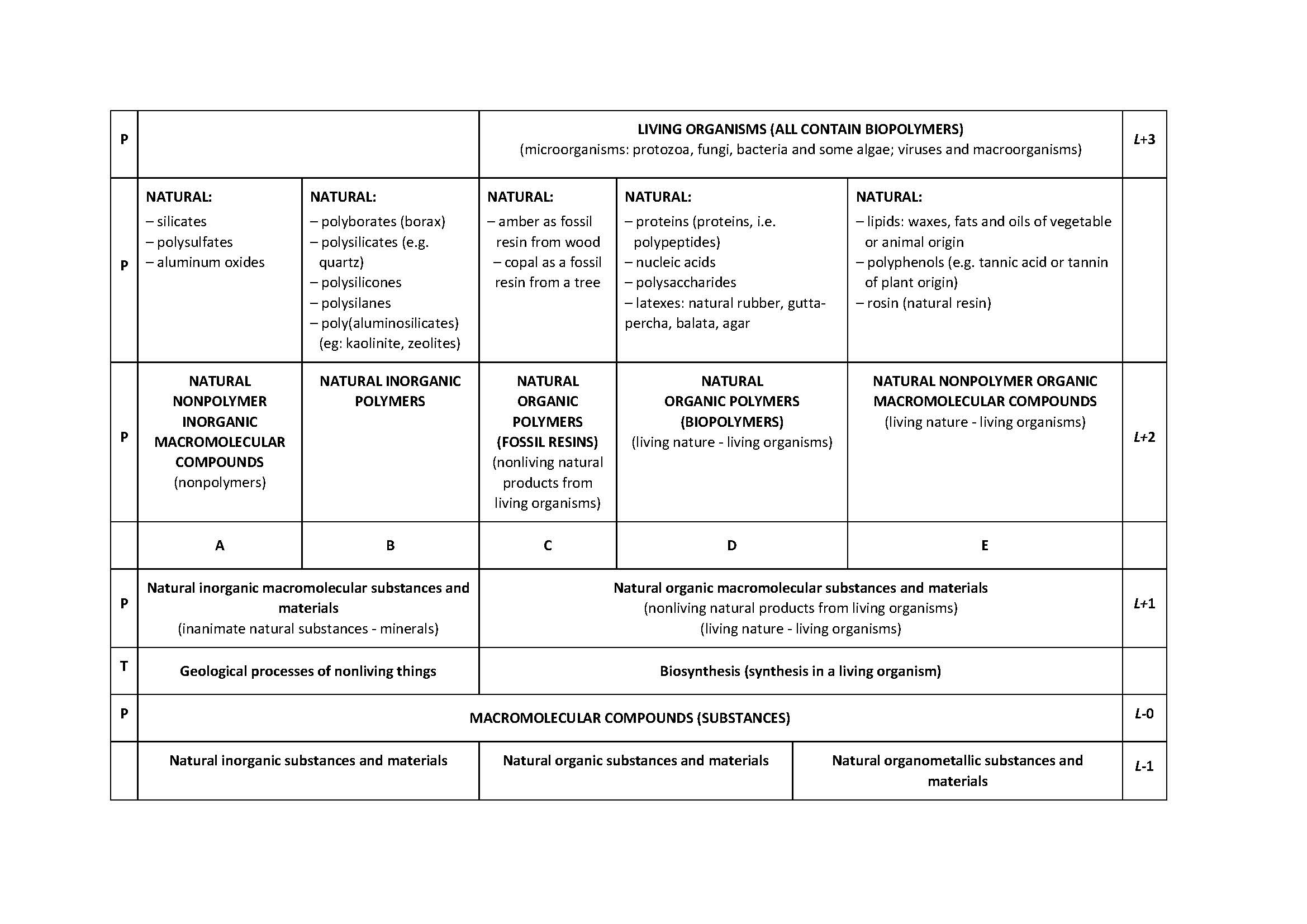
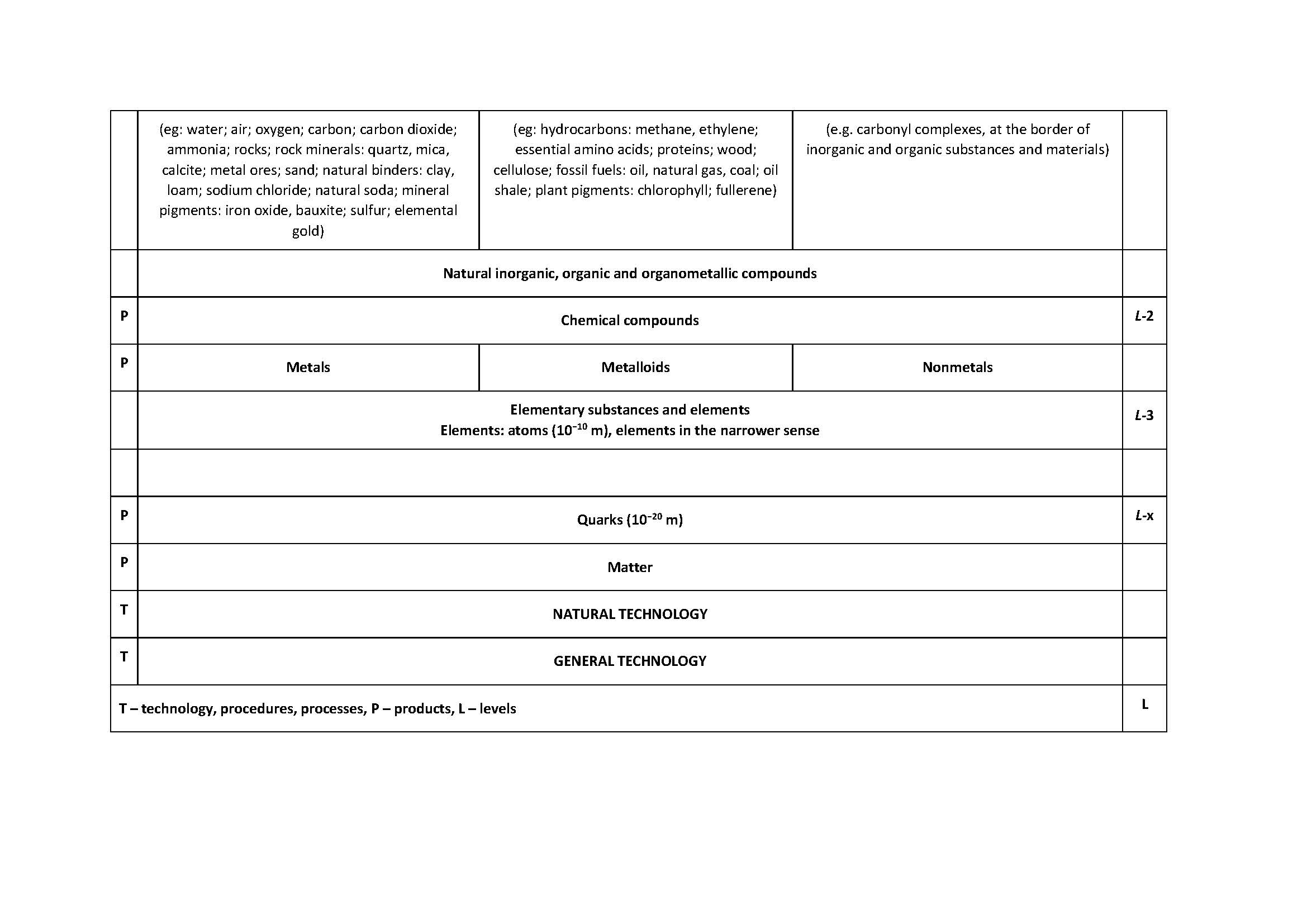
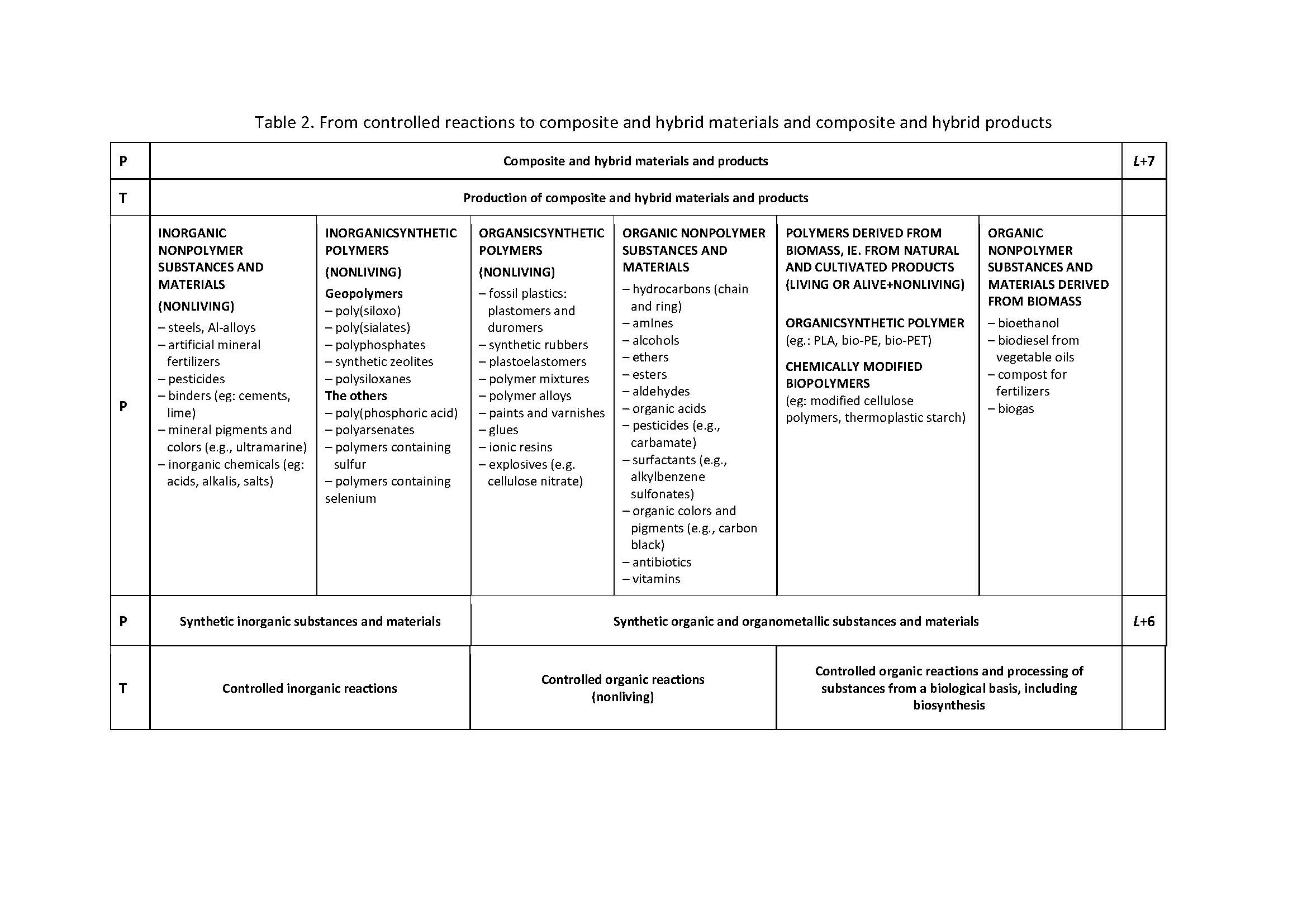
The article Polymers and nonpolymers - a new systematisation of substances and materials was published in the journal Rubber-Fibre-Plastics in 2014. Over time, it was found that it is possible to improve the systematisation by introducing three levels. These are: R-3: Elemental substances and elements, R-2: Chemical compounds and R-1: Natural inorganic, organic and organometallic compounds. The level R + 6 has been significantly expanded: Synthetic inorganic substances and materials and Synthetic organic and organometallic substances and materials. It proved necessary to define several terms from which polymers, polymer and polymerisate stand out.
Introduction
The number of substances and materials is constantly and rapidly growing. From chemical compounds built from atoms to those that exist only as products of these materials (e.g. rubber and ceramic products). The systematisation of substances and materials is becoming more and more necessary, even from the level of atoms. This would achieve two goals. First, their affiliation to certain basic groups would be better noted, whether they belong to inorganic or organic polymers or inorganic or organic nonpolymers. Another necessity is to become aware ofthe difference between natural and artificial substances and materials.
The starting point for expanding the systematisation of substances and materials is the article from 2014 [1,2] which was somewhat expanded in [3] in the Croatian version. Table 1 shows the expanded supplementary table from the papers [1-3]. The extension refers to the introduction of the level of elementary substances and elements - particles at the atomic level (10-10 m) (R-3 level) and to the introduction of the level of natural inorganic, organic and organometallic compounds, as well as substances and materials based on these compounds (level R-1). The divisions at the R + 2, R + 4 and R + 5 levels have been expanded. The level of R + 6 is described in much more detail in Table 2. The divisions of synthetic inorganic and organic polymeric and nonpolymeric substances and materials have been expanded andthe most important terms were defined.
Definitions
Matter, substance and chemical substance
Today, matter in general is divided into substances (matter in the classical sense) and energy, which are interconnected by the relations of the theory of relativity and quantum mechanics. Substance, as matter in the classical sense, is characterised by mass [4].
A chemical substance is defined as a matter of constant composition that is best characterized by the entities (molecules, atoms) it is composed of. Physical properties such as density, refractive index, electric conductivity, melting point, etc., characterise the chemical substance [5].
According to REACH, a substance is a chemical element and its compounds in the natural state or obtained by any manufacturing process, including any additive necessary to preserve its stability and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition [6].
A substance which occurs in nature is a naturally occurring substance as such, unprocessed or processed only by manual, mechanical or gravitational means, by dissolution in water, by flotation, by extraction with water, by steam distillation or by heating solely to remove water, or which is extracted from air by any means. [6]
A substance that has not been chemically modified is a substance whose chemical structure remains unchanged, even if it has undergone a chemical process or treatment, or a physical mineralogical transformation, for instance to remove impurities [6]
Atoms, elements and elementary substances
The atom is the smallest particle still characterizing a chemical element. It consists of a nucleus of a positive charge (Z is the proton number and e the elementary charge) carrying almost all its mass (more than 99.9%) and Z electrons determining its size. [5]
The term element has two meanings, elementary (simple) substance and element in the narrower sense, which has not only a practical but, even more, a principled, philosophical meaning. [7] An elementary substance is a pure substance with a homogeneous composition, while the term element indicates the possibility of chemical combination. [7]
According to IUPAC, a chemical element is: 1. A species of atoms; all atoms with the same number of protons in the atomic nucleus. 2. A pure chemical substance composed of atoms with the same number of protons in the atomic nucleus. Sometimes this concept is called the elementary substance as distinct from the chemical element as defined under 1, but mostly the term chemical element is used for both concepts. [5]
A chemical element is a set of all similar atoms in nature (space), which have the same number of protons in the nucleus. Chemical elements can have more isotopes, so a chemical element is a set of atoms of one or more isotopes. A chemical element cannot be broken down into simpler atoms by chemical methods. [8]
Chemical elements can be metals, metalloids, and nonmetals. [9] They occur in all three aggregate states: solid (all metals except mercury), liquid (e.g., mercury) and gaseous (e.g., oxygen).
Until 2014, 114 elements were included in the periodic table, of which those with atomic numbers 1 to 98 were found in nature, and those with atomic numbers 99 to 112, 114 and 116 were synthesized in the laboratory and confirmed by IUPAC. Synthesis was published for elements 113, 115, 117 and 118, but they were not confirmed by IUPAC. [10]
Metals are chemical elements made up of atoms that easily change into positive ions. Metals are chemical elements in a solid aggregate state, except for mercury, which is liquid, they have a characteristic luster, malleability, and ductility, and they are good conductors of heat and electric current. [10]
Nonmetals are a group of electronegative chemical elements to which belong: halogen elements, noble gases and hydrogen, carbon, nitrogen, oxygen, phosphorus, sulphur and selenium. Nonmetals are chemical elements of low density and malleability, pronounced ionisation properties and high electronegativity. They do not conduct electricity (except for graphite), and they come in gaseous, liquid, and solid aggregate states. [10]
An elementary substance is a macroscopic sample of a substance that consists of identical atoms, that is, of atoms of a certain element. Elementary substances of the same element can differ substantially in their properties, which is a consequence of different bonding of similar atoms. [11]
To date, 118 types of atoms of chemical elements are known, with over 600 of their elemental substances. [8] The chemical element carbon has several forms of elementary substances, and of several allotropic modifications, the two most famous are: diamond and graphite. Diamond and graphite are elementary substances of the same chemical element, and they can be converted into each other.
Chemical compounds are complex pure substances of constant composition, built from atoms of different elements. [12] In terms of physical and chemical properties, chemical compounds differ significantly from the elements of which they are composed. Chemical compounds are natural or synthetic in origin, inorganic, organic or organometallic in composition. [12] Organometallic compounds are compounds that have bonds between one or more metal atoms (boron, silicon, arsenic, selenium) and one or more carbon atoms from the organic group. [5]
A chemical compound is also defined as a substance that is formed by the chemical reaction of two or more substances. According to the type of chemical bond, compounds can be ionic (e.g., sodium chloride or NaCl), covalent (e.g., polymeric) and complex (e.g., metal carbonyl). [10]
A chemical reaction is a process in which a new chemical substance (or more of them) is formed from chemical substances. Synthesis is the process of creating a complex product from simple reactants. [10]
More than 20 million chemical compounds are known today, and new ones are synthesized every day. Four fifths of the Earth is covered by one of the simplest chemical compounds - water, and most solid substances in nature are mixtures of chemical compounds (wood, soil, rocks). Living organisms consist of water and other chemical compounds, and the main ingredients of food are organic compounds (proteins, fats, carbohydrates). However, only about 1% of the known number of compounds currently has market applications, and they are called chemicals. [12]
Organic compounds [13] An organic compound is a chemical compound that contains carbon (with certain exceptions), and all other compounds belong to inorganic compounds. Organic compounds are not only compounds found in the living world, but many of them are also synthesized artificially in the laboratory and are not found in living organisms.
One of the criteria for division can be compounds found in nature or obtained artificially.
Natural compounds refer to those produced in plants or animals. Many of them are still obtained from natural sources because it would be too expensive to produce them artificially. Examples of these compounds are most sugars, some alkaloids and terpenoids, certain nutrients such as vitamin B12. Other compounds that are important in biochemistry are: antigens, carbohydrates, enzymes, hormones, lipids and fatty acids, peptides and amino acids, lectins, vitamins and edible fats and oils.
Synthetic compounds refer to compounds obtained in the laboratory or under operational conditions, by reaction between other compounds. These can be compounds that have already been found in plants or animals or compounds that are not found in nature.
Most polymers (which include plastics and elastomers) are organic compounds.
Macromolecule (polymer molecule), macromolecular, polymeric
According to IUPAC, the definition of the term ‘macromolecule’ includes the definition of the terms ‘macromolecular’ and ‘polymeric’ which are used as adjectives. [5]
1. Macromolecule is a molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, molecule can be regarded as having a high relative molecular mass if the addition or removal of one or a few of the units has a negligible effect on the molecular properties. This statement fails in the case of certain macromolecules for which the properties may be critically dependent on fine details of the molecular structure.
2. If a part or the whole of the molecule has a high relative molecular mass and essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass, it may be described as either macromolecular or polymeric, or by polymer used adjectivally.
Polymers
Several definitions of polymers are given. It is emphasized that the word polymers are the necessary name for the highest or peak term.
It makes sense to use the name polymers as a collective name for natural and synthetic substances and materials whose main ingredient is a system of macromolecules (polymeric molecules). [14]
Polymers are chemical compounds or mixtures of compounds formed by polymerization and consisting essentially of repeating structural units. [15]
Polymers are substances composed of macromolecules, very large molecules with molecular weights ranging from a few thousand to as high as millions of grams/moles. [16]
REACH defines polymer as a substance consisting of molecules characterised by the sequence of one or more types of monomer unit. Such molecules must be distributed over a range of molecular weights. Differences in the molecular weight are primarily attributable to differences in the number of monomer units. [17]
A polymer is defined as a substance meeting the following criteria: [17]
(a) Over 50 percent of the weight for that substance consists of polymer molecules and,
(b) The amount of polymer molecules presenting the same molecular weight must be less than 50 weight percent of the substance.
In the context of this definition, a "polymer molecule" is a molecule that contains a sequence of at least 3 monomer units, which are covalently bound to at least one other monomer unit or another reactant, and a "monomer unit" means the reacted form of a monomer substance in a polymer. [17]
According to their origin, polymers can be natural or synthetic, and according to their chemical composition, organic or inorganic.
A polymer is obtained from a monomer, a molecule that can react with other monomer molecules forming a longer polymer chain or a three-dimensional network, through the polymerisation process. [14]
If the polymerisation product does not contain additives, it is called polymerisate. [12]
The duality of the word polymer
There is a duality of the word polymer in the English language. That is why it is suggested that the word polymers be used as a general, highest, or peak term. In that case, it would be appropriate to use the word polymerisate for the product of polymerisation in accordance with the above definition.
Imprecise statement
Very often the following sentence is encountered: ‘Polymers is a common name for plastics and rubber’. In a certain context, in texts dealing only with plastics and rubber, the sentence is acceptable. However, it is imprecise on its own. ‘All plastics and rubber are polymers, but all polymers are not just plastics and rubber.’
Biosynthesis and biopolymers [5]
Biosynthesis is the production of a chemical compound by a living organism.
Biopolymers are macromolecules (including proteins, nucleic acids, and polysaccharides) formed by living organisms.
Natural polymers [18]
Natural polymers are considered as the result of a polymerisation process that took place in nature, regardless of the extraction process through which they were extracted. This means that natural polymers are not necessarily ‘naturally occurring substances’ when assessed against the criteria set out in the REACH Regulation.
Another key difference is whether the polymerisation process occurred in nature or is the result of an industrial process involving living organisms. Based on the REACH Regulation and the related ECHA (European Chemicals Agency) Guidelines, polymers produced by the process of industrial fermentation are not considered natural polymers because the polymerisation did not occur in nature. Therefore, polymers that are created by biosynthesis in artificial cultivation and fermentation processes in an industrial environment, for example poly(hydroxyalkanoates) – PHA, are not considered natural polymers, because they are not the result of a polymerization process that occurred in nature. If a polymer is obtained through an industrial process, and the same type of polymer exists in nature, the manufactured polymer is not considered a natural polymer.
Biologically based products
The term ‘biomass products’ refers to products that are wholly or partially derived from biomass, such as: plants or animals. Biomass can be subjected to physical, chemical, or biological treatment. Biomass is material of biological origin, excluding materials found in geological formations and/or as fossils. Example is cellulose, but also plastics like PLA. [19]
Standard EN 17228:2019 is relevant for plastics obtained from biomass. [20]
Composites and hybrid polymers
A composite is a multicomponent material consisting of several different (non-gaseous) phase domains in which at least one type of phase domain is a continuous phase. Foam, which is a multiphase material consisting of a gas dispersed in a liquid or solid, is not usually considered a composite. [5]
A hybrid polymer is a polymer or polymer network consisting of inorganic and organic components. Examples include inorganic-organic polymers and organic-inorganic polymers. [5]
Conclusions
The division of substances and materials into polymers and nonpolymers, described in [1-3], is expanded for the level of elementary substances at the level of atoms and for the level of natural inorganic, organic and organometallic compounds, as well as substances and materials based on these compounds. The divisions at levels L+2, L+4 and L+5 have been expanded. The division of synthetic inorganic and organic polymeric and nonpolymeric substances and materials has been expanded. Definitions of the most important terms, vital for the understanding of tables 1 and 2, which were not presented in this form in previous works, are given. [1-3] The distinction between the words polymers and polymer is made and the possibility of using the word polymerisate is indicated.
Addition
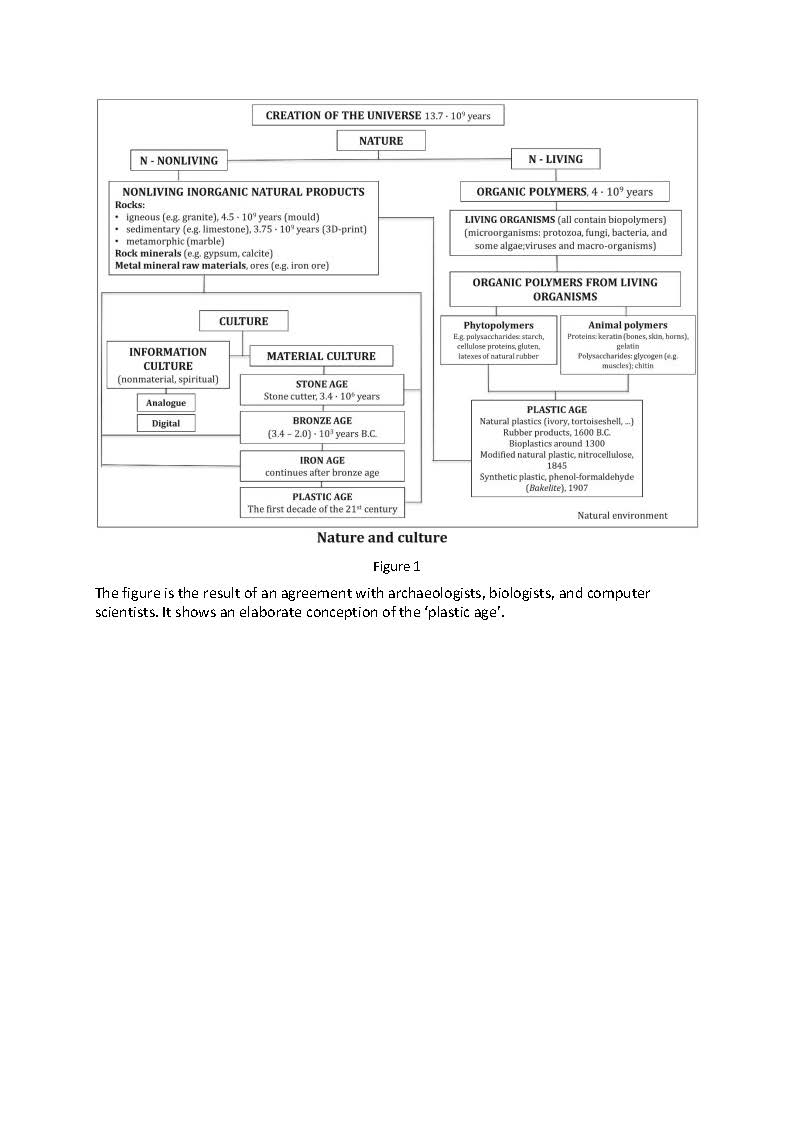
After the publication of the Croatian version of the text, Figure 1 was developed for the purposes of the lecture 115 Years of Synthetic Plastics. [21]
The figure is the result of an agreement with archaeologists, biologists, and computer scientists. It shows an elaborate conception of the ‘plastic age’.
References
- Čatić, I., Barić, G., Rujnić-Sokele, M.: Polymers and non-polymers – a new systematisation of substances and materials, Rubber-Fibre-Plastics 9(2014)1, 50-57.
- Čatić, I., Barić, G., Rujnić-Sokele, M.: Polymers and non-polymers – a new systematisation of substances and materials, Polimeri 36(2015)1-2, 15-22. (identično s1).
- Čatić, I.: Proizvodnjamaterijalaiproizvodnjatvorevina, Svetpolimera 23(2020)1–2, 11–15.
- Materija. Hrvatska enciklopedija, mrežnoizdanje. Leksikografskizavod Miroslav Krleža, 2021. Accessed 18. 8. 2021. <http://www.enciklopedija.hr/Natuknica.aspx?ID=39401>.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaughtand A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook.
- REGULATION (EC) No 1907/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European ChemicalsAgency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 andCommission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC
- Raos, N.: Elementi I elementarne tvari, Kem. Ind. 68(7-8)2019)317-322.
- https://hr.wikipedia.org/wiki/Kemijski_element 290721
- Periodni sustave lemenata, Hrvatska enciklopedija, mrežn oizdanje. Leksikografski zavod Miroslav Krleža, 2021
- Kemija – Struna, Hrvatsko strukovno nazivlje, Institut za hrvatski jezik I jezikoslovlje, http://struna.ihjj.hr/browse/?pid=12, accessed 18. 8. 2021.
- Elementarna tvar, Hrvatska enciklopedija, mrežno izdanje. Leksikografski zavod Miroslav Krleža, 2021. Accessed 19. 8. 2021. <http://www.enciklopedija.hr/Natuknica.aspx?ID=17682>.
- Kemijski spojevi, Hrvatska enciklopedija, mrežno izdanje. Leksikografski zavod Miroslav Krleža, 2021. Accessed 18. 8. 2021. <http://www.enciklopedija.hr/Natuknica.aspx?ID=31168>.
- https://hr.wikipedia.org/wiki/Organski_spoj, accessed 21. 8. 2021.
- Čatić, I.: Proizvodnja polimernih tvorevina, Društvo za plastiku I gumu, Zagreb, 2006.
- https://www.merriam-webster.com/dictionary/polymer
- https://iupac.org/polymer-edu/what-are-polymers/
- European Chemicals Agency – ECHA: Guidance for monomers and polymers, April 2012, Version 2.0 Guidance for the implementation of REACH.
- COMMISSION NOTICE – Commission guidelines on single-use plastic products in accordance with Directive (EU) 2019/904 of the European Parliament and of the Council on the reduction of the impact of certain plastic products on the environment (2021/C 216/01)
- EN 16575:2014: Bio-based products – Vocabulary.
- EN 17228:2019: Plastics– Bio-based polymers, plastics, and plastics products - Terminology, characteristics and communication.
- Čatić, I.: 115 godina sintetske plastike, Festival znanosti 22, TMNT, Zagreb, 2022-05-03.

