Sodium-based material yields stable alternative to lithium-ion batteries
Researchers hope new research can pave way for more productive battery technology.
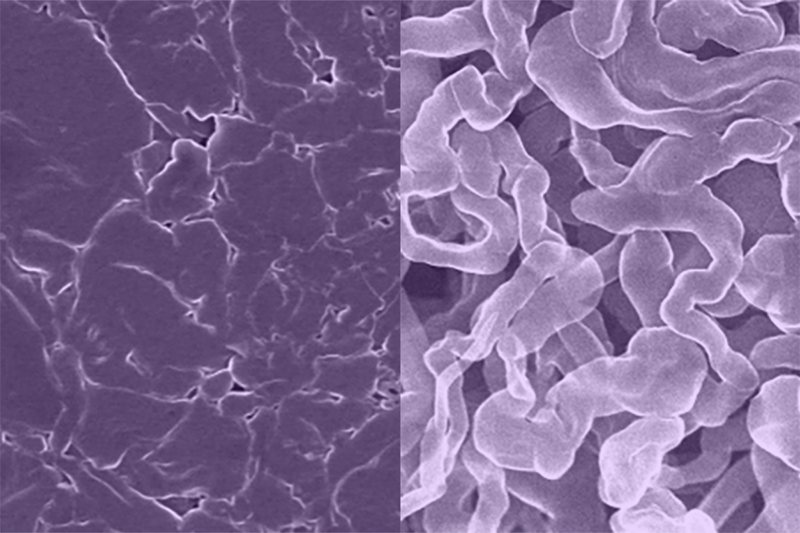
A team at the University of Texas, USA at Austin has created a new sodium-based battery material that is highly stable and capable of recharging as quickly as a lithium-ion battery.
In early sodium batteries, the anode would grow needle-like filaments called dendrites that can cause the battery to electrically short and even catch fire or explode. The new anode material, called sodium antimony telluride intermetallic – Na metal composite (NST-Na), solves this issue while providing a faster charge, the team claim.
‘We’re essentially solving two problems at once,’ says Professor David Mitlin at the University. ‘Typically, the faster you charge, the more of these dendrites you grow. So if you suppress dendrite growth, you can charge and discharge faster, because all of a sudden it’s safe.’
According to the researchers, this ‘sodium metal anode theoretically has the highest energy density of any sodium anode, says Professor Graeme Henkelman, also from the University.
The material is made by rolling a thin sheet of sodium metal onto an antimony telluride powder, folding it over on itself, and repeating many times.
This process results in a very uniform distribution of sodium atoms that makes it less likely to form dendrites or surface corrosion than existing sodium metal anodes. That makes the battery more stable and allows faster charging, comparable to a lithium-ion battery’s charge rate. It also has a higher energy capacity than existing sodium-ion batteries.
Henkelman explains that if the sodium atoms that carry a charge in a sodium battery bind more strongly to each other than they do to the anode, they tend to form instabilities, or clumps of sodium that attract more sodium atoms and eventually lead to dendrites.
The group hopes the new materials can pave the way towards delivering more energy than current battery technologies.







