Smart approach to dental care powers tissue rejuvenating light
A dental implant that can resist bacterial growth and generate its own electricity from brushing and chewing to power a gum-tissue-rejuvenating light, is under development.
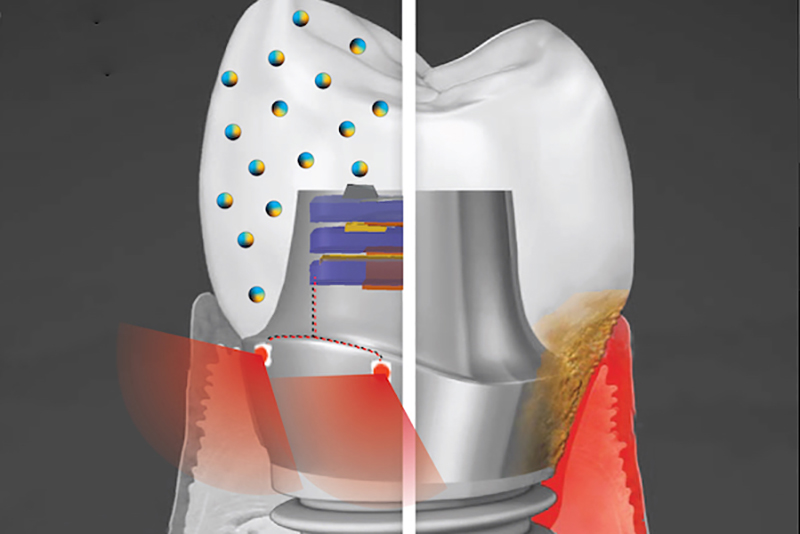
A smart dental implant employing biofilm-resisting nanoparticles and light powered by biomechanical forces to promote health of the surrounding gum tissue
© Albert KimThe implant system contains an energy-harvesting and bacteria-repelling crown with embedded piezoelectric nanoparticles. ‘By inhibiting colonisation of bacteria on implant (and implant-supported restorations – crown) and mitigating inflammatory gingival tissue response, the occurrence of peri-implant diseases can be reduced, while prolonging lifetime and improving function of the implant,’ explains Geelsu Hwang, Assistant Professor at the University of Pennsylvania, USA, School of Dental Medicine.
The second technology is an embedded light source for phototherapy, powered by the natural motions of the mouth, such as chewing or toothbrushing. Microelectronic circuits and red or near-infrared light-emitting diodes are embedded in the titanium abutment.
‘Phototherapy can address a diverse set of health issues,’ says Hwang. ‘But once a biomaterial is implanted, it’s not practical to replace or recharge a battery. We are using a piezoelectric material, which can generate electrical power from natural oral motions to supply a light that can conduct phototherapy, and we find that it can successfully protect gingival tissue from bacterial challenge.’
The researchers have used barium titanate (BTO) for its piezoelectric properties, conventionally exploited in capacitators and transistors, but until now has not been explored as a foundation for anti-infectious implantable biomaterials.
The nanoparticle-infused material is created by mixing nanopowder and dental resin, followed by thoroughly stirring it overnight on a roller mixer. ‘After overnight stirring, mixtures were degassed,’ Hwang adds. ‘Then, samples were cured under ultra-violet light and subjected to sintering. To decompose electric dipoles, so that an externally applied strong electric field can align them, BTO-nanocomposites were subjected to the poling process.
‘The poling stage with samples was submerged in a silicone oil bath while heated near Curie temperature of BTO (>80°C). Using the poling stage and a high-voltage source, a uniform electric field of 1kV/mm was applied across the sample for two hours.’
To test its potential, the team has used discs embedded with nanoparticles of BTO and exposed them to Streptococcus mutans – a primary component of the bacterial biofilm responsible for tooth decay, commonly known as dental plaque. They have found that the discs resist biofilm formation in a dose-dependent manner. Discs with higher concentrations of BTO are better at preventing biofilms from binding, with the anti-adhesive effect leading to ~10-fold reduction in colony-forming units in vitro.
While earlier studies suggested that BTO might kill bacteria outright using reactive oxygen species generated by light-catalysed or electric polarisation reactions, the team did not find this to be the case due to the short-lived efficacy and off-target effects of these approaches. Instead, the material generates enhanced negative surface charge that repels the negatively charged cell walls of bacteria. It is likely that this repulsion effect would be long-lasting, the researchers say.
Hwang continues, ‘BTO-nanocomposite does not have bacterial killing activity, so it would minimise antibacterial resistance nor disrupt the homeostasis of the oral microbiome. Inhibition of bacterial colonisation occurs due to the physicochemical properties of bacteria and BTO-nanocomposites which generate repulsive interaction energy between them.’
The team reports that the implant system can successfully block biofilm formation on crown material, as well as boost gingival tissue cell to resist bacterially-induced cell inflammation.
The power-generating property of the material is sustained, and in tests over time the material did not leach. It also demonstrates a level of mechanical strength comparable to other materials used in dental applications, and the material is said to be harmless to normal gingival tissue, supporting the idea that this could be used without ill effect in the mouth.







