Safer lithium battery electrolytes
Adding alkali metal additives to lithium battery electrolytes could boost efficiency and safety credentials.
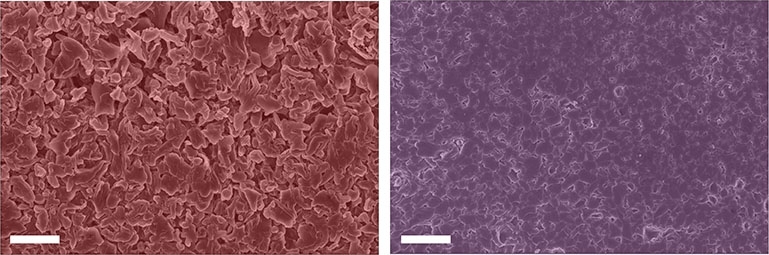
Potassium salt can put the brakes on microstructure formation in lithium metal batteries, claims a team from Columbia University’s School of Engineering and Applied Science, USA. In doing so, this could enhance the device’s efficiency and prevent it from catching fire.
‘In this work, we tested the addition of small amounts (10-15%) of potassium to the lithium battery electrolyte. We found that this prevented short circuiting of the battery by stopping electrolyte decomposition at the lithium metal surface,’ says Lauren Marbella, Assistant Professor of Chemical Engineering at the University. ‘We were actually able to determine what chemicals the electrolytes were breaking down into and found that potassium prevented the formation of poor ion conductors on the lithium metal surface.’
Marbella explains that there is significant interest in moving away from commonly used graphite anode to lithium metal anodes to make batteries lighter and provide more range for electric vehicles, and so a solution to the formation of microstructures is important for this transition.
She adds that there is currently ‘no “good” electrolyte to use with lithium metal batteries. Most electrolytes are a highly complex mixture of organic solvents and salt. The addition of small amounts of potassium salt to existing lithium battery electrolytes is a cheap, easy way to improve the performance of lithium metal batteries and we understand better why it works, which makes it easier to make adjustments’.
Several other alkali metal additives, including rubidium and cesium, have also been investigated. ‘We have shown that potassium actually prevents the formation of undesired compounds in the surface layer on lithium that are linked to microstructure growth. Some preliminary calculations from our collaborators at Carnegie Mellon University [USA] suggest that rubidium and cesium probably behave the same way, but of these three alkali metals, potassium is the cheapest – [even] cheaper than lithium,’ Marbella says.
To verify the new approach, the team performed electrochemical tests to measure battery efficiency, electron microscopy to learn how potassium additives changed lithium microstructural growth patterns, nuclear magnetic resonance spectroscopy to look at the extent and type of electrolyte degradation, X-ray spectroscopy and mass spectrometry to determine where the potassium ends up during battery operation, and density functional theory calculations to understand how potassium modulates reactivity with the electrolyte at the lithium metal surface.
The team is now working on incorporating potassium additives into more sophisticated electrolyte formulations to further improve performance, as well as enhance understanding of why electrolytes decomposition works differently in systems containing potassium compared to lithium.
Before commercialisation, rigorous testing of how the batteries perform with more highly optimised electrolye formulations is needed, concludes Marbella.







