Patent of the month – ionic conducting compositions for energy storage devices
Dr Tahsin Ali Kassam at UDL Intellectual Property discusses a recently granted patent relating to ionic conducting compositions for energy storage devices.
The need for high energy density lithium-ion cells has placed increasing demand on the performance of separators – located between two electrodes as a layer immersed in liquid electrolyte that is electrically insulating and permeable to lithium ions. Separators are required to maintain their permeability while being sufficiently thick to prevent short circuits caused by dendritic growth of lithium metal. However, with liquid electrolytes that are usually flammable carbonates, there is always some frequency of catastrophic failure.
An increasingly popular alternative to lithium-ion cells with liquid electrolytes are those incorporating solid-state electrolytes. These allow the use of lithium metal as an electrode with the only risk of dendritic growth being a ‘soft short’, i.e. a cell failure, but without an associated fire. Solid-state electrolytes replace both the liquid electrolytes and the separators. Several categories of solid-state electrolyte materials have merit, the most common being oxides, sulphides and polymers, although challenges for oxides and sulphides include reactions with air and moisture. Cells including polymers need to be operated above room temperature and charged slowly.
In June 2020, Morgan Advanced Materials plc was granted UK patent GB2568613B titled Energy storage device and ionic conducting composition for use therein, which claims an energy storage device comprising a melt-formed glass or glass ceramic silicate composition with a formula LivM1wAlxSiyOz, where M1 is selected from the group consisting of alkaline earth metals, Ti, Zr, Ce, La, and combinations thereof; v, y and z are greater than 0; w is greater than 0; x is 0 or greater than 0; y ≥ x; and wherein LivM1wAlxSiyOz accounts for at least 95wt.% of the composition and wherein the lithium content (expressed as Li2O) is less than 15 wt.% of the composition. Optionally, M1 may include Zr, which was found to enhance the ionic conductivity of the composition.
The silicate composition is melt formed from a molten mass using techniques including those suitable for forming fibres such as bushing, blasting, blowing and spinning. These processes, in which the silicate composition is formed as fibres or nanowires, allows for the manufacture of these materials on a larger scale as compared to that of conventional materials made using sol-gel or similar techniques.
The patent discloses a series of experiments performed to evaluate the suitability of selected compositions produced by spinning, in which a molten stream is formed and converted into fibre either by permitting the stream to contact one or more spinning wheels or by using an air blast directed at the stream. The results show that the fibre shrinkages, fibre diameters and melt crystallinity formed samples that were suitable for use in energy storage devices. In particular, the melt produced samples that exhibited significantly higher ionic conductivities as compared to sol-gel counterparts.
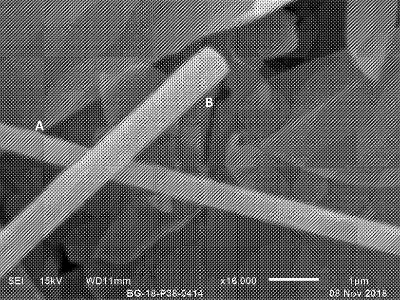
The silicate compositions may be used to form separators or composite electrolyte membranes. In one example, the electrolyte membrane (or matrix) was formed using Li2ZrSi6O15 fibres (figure shown above) that were added to a polyacrylonitrile-LiClO4 solution, creating a mixture that was cast onto a glass substrate and dried in a vacuum oven. The resulting membrane was placed between stainless steel plates and ion conductivity measurements were carried out using A.C. impedance spectroscopy over a frequency range of 1-10MHz in the temperature range of 22-80°C. The results show a significant improvement in the ion conductivity of the composite electrolyte membrane as compared with those of electrolyte membranes reported in literature studies.
This material is the first known with a high enough conductivity for use as a stand-alone electrolyte or within a polymer matrix in solid-state electrolyte cells. It is also stable for processing in air and can be scaled-up to mass production at an acceptable price to the lithium-ion battery industry.
Read the full patent at bit.ly/32PYqaz







