Packing in protection – pharmaceutical packaging during COVID-19
Gregor Anderson FIMMM at Pharmacentric Solutions, UK, on the critical role of pharmaceutical packaging during a pandemic and why the sector has an opportunity to boost its sustainability credentials.
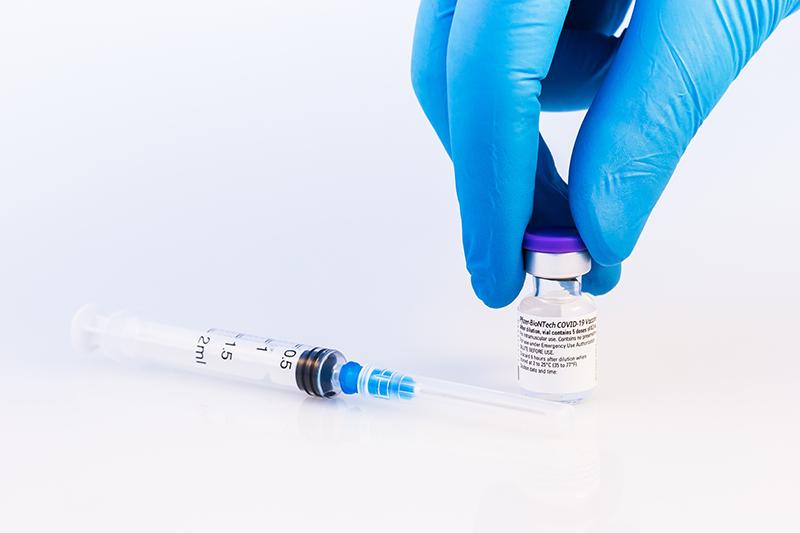
COVID-19 has highlighted to the world the criticality of aligning medical advancements with the right packaging to enable effective and lean supply.
Cold-chain requirements have been a differentiator with the Oxford/AstraZeneca vaccine, which does not require the more complex -70°C storage conditions of the Pfizer-BioNTech version. Keeping supply chains simple has been key to vaccine delivery using known heritage packaging components such as vials, stoppers, syringes and needles. This is the best option to ensure timely delivery, and the challenge now is managing the ongoing demand for these components to meet the growing volumes.
Longer term component capacity will need to be reviewed and updated to meet ongoing demand across the world. Affordability will be important, and this will be improved if production is highly efficient and volumes remain high.
There is potential for standardisation of COVID-19 vaccine packaging, but this will depend on a range of factors including temperature, carton size, method of dispensing and vial form. For the UK, it will be important to have a longer term in-country supply of vaccine packaging components as well as lean and secure vaccine manufacturing capacity.
Protecting and dispensing
Of all the packaging formats available today, pharmaceutical packs are one that has been available for many years, with the prime function of protecting and dispensing medicines while meeting complex regulations, performance requirements and the demands of long global supply chains along the way from factory to patient.
The packaging formats come in a wide range of platforms and are often specialised in the way they are specified, manufactured, assembled, filled and sealed.
Medicinal products extend across these various formats to include tablets, liquids, gels and powders, for example, and they are marketed and distributed globally. The typical cost of developing and commercialising just one new medicine now exceeds US$1bln, so the value of any approved medicine is high and making sure it reaches the patient securely is vital.
As such, the packaging and protection of medicines is critical to the successful end-to-end delivery, while also ensuring patients can take their medication easily, quickly and safely.
Pharmaceutical packaging specialists require an in-depth knowledge of materials, processes and the complex end-to-end supply chains used to package each specific medicine. Materials must obviously be robust and stable in use throughout the intended shelf life. The materials specified must also meet required quality standards and be capable of high-volume production, as most medicines are manufactured and packed in millions (if not billions) of doses to meet global demand.
These materials and processes are chosen with considerable care to meet specifications, and packs are extensively tested to validate each pack format prior to commercialisation.
Understanding the test requirements and processing parameters ensures that the appropriate design, material and process choice are optimised when manufacturing medicines at these high volumes. Packaging specialists and specifiers must also understand the medicines being packaged and the needs of the patient as disease areas do differ.
For example, packaging for an arthritis medicine will have to consider the dextrous limitations a patient may have when opening the pack that such a patient may have. This extensive need to have a wide awareness of manufacturing, materials, regulations and processing for a wide range of medicine platforms, as well as a knowledge of patient needs and medicine manufacturing makes the packaging specialists role a critical one. Furthermore, there is the need to ensure that updated standards, new innovations and technology enhancements are understood, and implemented as required.
Three formats
Pharmaceutical packaging is essentially split into three different formats. Each of these has different demands placed on the materials and design used.
Primary packaging
This is the pack material that is in direct contact with the medicine. Examples include a polymer or cold form blister or bottle for tablets, a vial or syringe for sterile liquids, or a tube for a dermatological cream, and even an aluminium can for an inhaler. Primary packs are typically labelled/coded with information such as batch number/product/expiry date, etc.
All these formats must undergo stability testing to ensure that the sealed primary pack meets an appropriate shelf life – typically 24-36 months – to offer robustness in the supply chain and also that there is no impact from material/medicine interaction – via extensive extractables and leachables testing. Safety attributes, such as child-resistant features, can be added to the primary packaging (and secondary packaging) as required.
Secondary packaging
Most medicine primary packs are placed into secondary packs. These are typically cartons but also extend to devices such as inhalers and injectors, which themselves are then placed into cartons. Additional secondary packaging may also be added to offer enhanced protection and these can include preformed polymer trays or foil overwrap.
Secondary packs are printed and labelled, and extensive transit testing is undertaken with finished packs to ensure that they are robust. Patient Instruction Leaflets (PILs) are also included with every pack as these are a regulatory requirement in many markets.
Tertiary packaging
This includes shipping cases, pallets and specialist formats – for example, those used in the cold chain – which is required for some medicines, such as vaccines.
Each of these pack formats mentioned offer future opportunities and are impacted by ongoing challenges, such as sustainability.

A healthy approach to sustainability
Medicine packaging is typically disposable, with much of the used material being placed in domestic waste that ends in landfill.
As detailed, materials used in packaging are chosen to meet requirements for performance, processing and cost. Sustainability is becoming an additional metric, but this has an associated set of risks as well as real benefits. As an example, blister packaging for tablets has traditionally been polymer based, with PVC a popular base material with an aluminium foil lidding material to ensure ease of accessibility. These blister packs are currently not suitable for recycling.
Bottles for tablets are a second option and are typically manufactured from high-density polyethylene so are more suitable for recycling. However, blister packs have the advantage of allowing the patient to see how many tablets they have taken and how many they have left, therefore assisting patient adherence and each individual tablet is protected.
Governments, health authorities and patients have increasing expectations to minimise the environmental impact of packaging. By default, medicinal packaging is designed to be functional, so it is often optimised from the outset. Where change is being seen is the specification of materials and the potential for packs to be more recyclable.
Risks of this approach for heritage medicines include Pharma companies having to undertake (essentially redo) stability testing – including extractables and leachables testing – invest in new processing equipment/tooling and also having to update all regulatory dossiers as packaging forms part of every drug submission. Manufacturing speeds may be reduced, and this could impact pricing and ultimately access to medicines.
However, there is the opportunity to create refined standards for primary pack formats for new medicines. The use of PET for blister bases and a trend towards the more recyclable bottle could be future options.
With the more worrying fact being that 50% of medicines are not taken effectively, this is a substantial sustainability opportunity. Not taking a medicine as prescribed will mean an ineffective outcome for the patient and this is the biggest waste.
Couple this with the risk of potential contamination from used medicine packaging, a more innovative approach for medicine packaging sustainability is needed. Take-back schemes offer the opportunity to collect medicine packaging and enable the patient to discuss any compliance issues they may have with the pharmacist/healthcare professional. These types of schemes have been initiated in countries like France and ensure medicine packaging can be collected safely and securely.
While take-back schemes are a more sustainable option, medicine packaging specifiers and suppliers are using sustainability design tools to optimise formats. These include light-weighting packaging and devices, improved processing and inspection technology to avoid production waste, using monomer materials to make recycling easier with some platforms, and using renewable materials from certified sources (FSC board and paper).
Looking further ahead, there could be industry-wide initiatives that have real patient benefits as well as sustainability ones. One such example is digitising the Patient Instruction Leaflets. With increased access to mobile phone and computer App platforms, these leaflets could be removed from packs. Yes, there would always be the need to supply a leaflet to those who don’t have digital access, but these could, for example, be printed on demand at the pharmacist.
The advantages of a digital leaflet are many and include being language specific – they could link to a video/voiced instructions (ideal for those with poor sight), be searchable, etc. Another hidden benefit is any artwork changes would be immediate. This, for example, would enhance safety if arising contraindications of a medicine are discovered and need to be included.
Pushing within limits
The Association of the British Pharmaceutical Industry's 2017 report, Manufacturing Vision for UK Pharma: Future proofing the UK through an aligned technology and innovation roadmap, lays out the opportunity to introduce different materials – mostly driven by challenges around sustainability.
Pharma has stringent requirements regarding materials and tends to utilise what is known, tried, and most importantly, tested. This does not preclude pharma from learning about what other industries are doing regarding packaging materials and adapting them for potential use.
An example of this is the use of a preformed aluminium tray to replace an overwrap. The tray enables the device packaging to be compact (optimised for transportation), easy to open by the patient (simple peel-back lid) and offers optimum shelf life due to its technical characteristics. An additional advantage is it is completely recyclable.
One constraint regarding primary pack materials is the regulatory requirement to use virgin materials. This is driven by the need to know the provenance of that virgin material. Currently, ensuring the provenance of a recycled material is not completely guaranteed. Minimising the use of the virgin material used is a core target for packaging specifiers and optimising processing by using CAD modelling and early development testing, for example, enables the right balance between material use and maximising shelf life.
Cool with wool
Woolcool, a company in Staffordshire, UK, has developed a range of insulated packaging using wool fibres. The firm suggests the packaging can be applied for pharmaceutical use as the material is effective at absorbing moisture from the air which minimises humidity and condensation, helping to maintaining stable temperatures.
Pharma is constantly looking for appropriate materials that offer patient and sustainability benefits, but these ultimately must not compromise performance. It learns from areas such as food and fast moving consumer goods (FMCG) packaging and these are areas that are typically faster to implement new innovations. One recent innovation that could benefit Pharma cold chains is the novel use of chicken feathers as an insulation material. It was developed by the start-up, Plummo, with the food delivery market as a key customer. The technology ultimately could be adapted to meet Pharma’s needs.
In training
Regulations must be followed with all Pharma packaging requirements. This extends across the primary, secondary and tertiary aspects of the specified pack. Recent initiatives, such as serialisation – where each pack has a unique referenceable identifier to ensure patient safety and pack traceability – have been implemented across Europe and beyond.
Packaging specialists must be aware of the impact of existing and proposed pack regulations and these vary across global markets. Sustainability regulations are being implemented with single-use plastics and packaging (FMCG)/food packaging and this could extend to medicines packing in the future. Understanding the impact and opportunity for this will be an expectation for the industry and the packaging specifiers and suppliers.
Designing and specifying the packaging used throughout the end-to-end supply for medicines is therefore a complex challenge. There are many stakeholders and considerations and a wide portfolio of medicine formats to consider – this includes novel platforms such as cell and gene therapy and Antibody Drug Conjugates. To ensure that packaging specialists (and those who specify, manufacture and procure packaging materials) remain aligned with new technologies and opportunities, ongoing training is a must.
The IOM3 Training Academy course, Packaging Principles for Pharmaceutical and Medical Technology, covers the specifications, the conversion processes and the impact of packaging choices through the supply chain. For more details go click here.


