Element focus CHROMIUM.pdf
The Basics
-
Chromium has atomic number 24 and atomic mass 51.99. It sits between vanadium and manganese and above molybdenum in the first transition series of the Periodic Table.
-
It has a density of 7.10gcm-3, melts at 1907°C, boils at 2671°C and is a body centred cubic solid at room temperature.
-
It is the 21st most abundant element in the Earth’s crust and occurs at an average concentration of 100ppm.
-
One of the first known uses of chromium dates back over 2000 years. The bronze tips of cross bow bolts belonging to the Terracotta Army of the Qin Dynasty in China were found to be coated in chromium which left them untarnished.
-
Its name comes from the Greek word chroma meaning colour because its compounds have a broad range of colours.

Occurence & Extraction


-
Chromium is a steel-grey lustrous metal which can take a high polish. It is relatively hard but malleable and many of its applications are due to its excellent corrosion resistance.
-
Chromium becomes rapidly passivated by oxygen when exposed to air. This stable oxide layer has a spinel structure and is only a few atoms thick, yet it is dense enough to prevent further diffusion of oxygen to the underlying material.
-
In modern times chromium was first discovered and extracted by Louis Nicolas Vauqueline in 1797 in the mineral crocoite (lead chromate).
-
Annual production of chromium amounts to around 4.5 million tonnes and 85% of this is used in alloying and plating.
-
The main ore of chromium is chromite, or FeCr2O4 and its main producers are South Africa, Kazakhstan, Turkey and India.
-
The process by which chromite is refined depends on the desired product. To make ferrochromium an electric arc furnace is used to reduce the chromite.
-
To produce the pure metal the iron and chromium must be separated; this is done in two stages. Firstly, the chromite is roasted in air with calcium carbonate and sodium carbonate. By following this with a high temperature leaching process the chromates are dissolved while the iron oxide remains insoluble. Sulphuric acid is used to first convert the dissolved chromate to dichromate; this is then converted to chromium oxide before being reduced to chromium with carbon.
-
The inclusion of a trace amount of chromium in corundum and beryl gives rubies and emeralds their characteristic red and green colour, respectively.
Structure & Properties
-
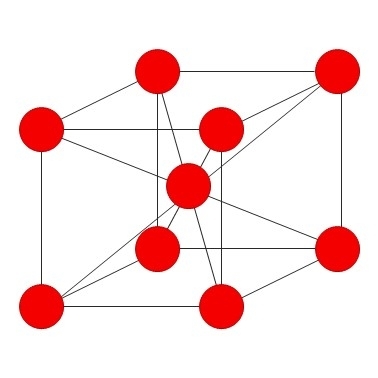
Chromium is ablue-silver metal that adopts a body centred cubic crystal structure.
-
It is the third hardest element after carbon (diamond) and boron.
-
Chromium reacts with oxygen in the air to form a stable passive oxide layer.
-
It forms a substitutional alloy with steel, providing strengthening by introducing strain into the crystal lattice.
Materials & Applications



-
Chromium is relatively expensive, costing around £6000 per tonne in 2019 (compared to £330 for iron, £1380 for aluminium and £4650 for copper)
-
Chromium is added to steels in the form of ferrochromium.
-
In tool steels a concentration of 3 to 5% by weight is added and stainless steels require the addition of around 18wt% chromium along with 8 to 10% of nickel. Stainless steels are extensively used for cutlery and kitchenware, in architecture, chemical engineering equipment and medicine.
-
It is also added to nickel-based superalloys where it forms stable carbide particles which provide grain boundary strengthening.
-
Electroplating with chromium provides a corrosion resistant coating which can be polished to a high shine. One of the most well know uses of ‘chrome’ coatings is on automotive components.
-
Chromium has many possible oxidation states (the 3+state is the most stable) and chromium compounds are used in many industries.
-
Pigments containing chromium compounds can be a variety of colours, including chromium yellow (based on lead chromate) which was for many years used to paint school buses.
-
Chromium sulphate is used in the leather tanning process; however, the waste is toxic so alternative methods are being sought.
-
Trace amounts of chromium are required for humans to remain healthy.







